Novel Antimicrobial Nanotechnologies
1. Background and state-of-the-art
Infections continue to be among the leading threaten to human life.1 In the same time, designing effective and long-lasting antimicrobial therapies is one of the most difficult tasks of modern medicine.2 When the delicate balance of bacterial ecosystem in the human organism is disrupted, pathogens become dominant and induce infections.3-5 Particularly complicated situation starts when planktonic pathogens start to colonize surfaces leading to biofilms- forms highly resistant to antibiotics.2 Prevailing antimicrobial therapies are based on biochemical stimuli (antibiotics, antimicrobial nanoparticles, antimicrobial ions, antimicrobial peptides etc.) with mechanisms that give a lot of possibility for bacteria to develop resistance.1,6 In contrast to them physical stimuli (thermal,7 mechanical,8 magnetic9 or electrical10) affect bacterial life in different way. Numerous studies have shown that defects in the bacterial membrane induced by the physical stimuli increase the susceptibility to biochemical stimuli.5 Therefore, the synergy between the physical and the biochemical stimuli could be particularly effective in targeting not only planktonic but also bacteria in biofilms and eliminating their chances to develop resistant forms. This opens interesting novel standpoints for designing innovative antimicrobial (nano)technologies.
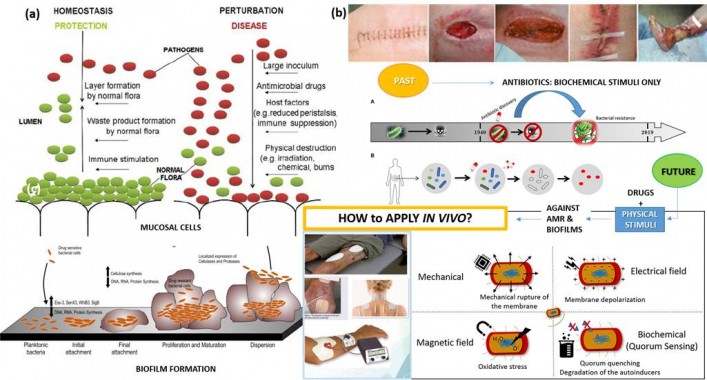
Figure 1: Bacterial microenvironment: perturbations causing infection and formation of biofilms (a);17 Stimuli affecting bacterial life: a pathway of developing a problem of antimicrobial resistance (AMR) in the past, possibilities and problems with combining physical stimuli with antimicrobials as a solution for the future (b).18
2. Objectives, originality and impact on new research approaches
The main objective of the proposed study is beyond state of the art research in designing novel generation of antimicrobials based on combination of physical and biochemical stimuli. Our intention is to prove that combination of physical stimuli (that mechanically destroy membrane) and biochemical stimuli (that affect different phases of bacterial life) will leave bacteria without any possibility to develop resistance. For that purpose we want to design novel type of biomaterials, novel contact-based antimicrobials, that use:
(i) A very high density of cationic surface charge to disrupt negatively charged bacteria membrane (hierarchically structured cationic, amino acid functionalized AuNPs containing cyclodextrines)
(ii) Piezostimulation for disintegration of bacterial cells using medical ultrasound (organic piezoelectrics based on hydrophilic piezo-PLLA surfaces)
(iii) Local photothermia to thermally decompose bacterial cells after illumination by laser (Ga-Au core-shells and ferrite-AuNPs structures).
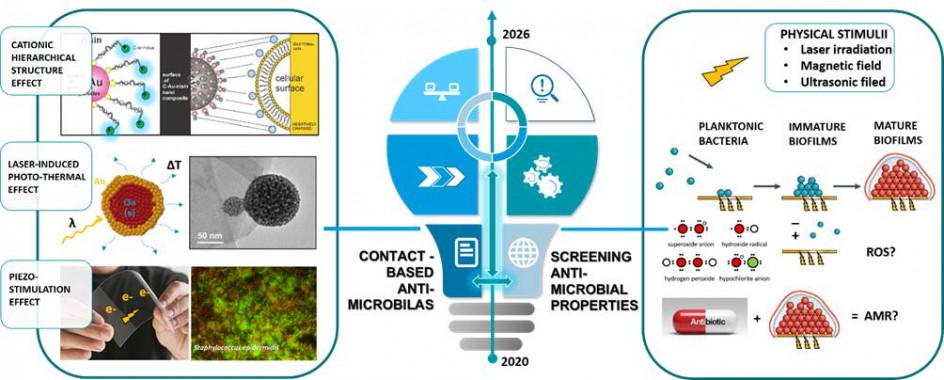
Figure 2: Illustration of the design of contact-based antimicrobials and testing their efficacy.
We are holding a patent on contact-based antimicrobials which will be the bases for further studies containing cationic AuNPs.11 Using hierarchically structured functionalized AuNPs we managed to tailor antimicrobial spectrum of nisin towards Gram negative bacteria, including antibiotic-resistant strains, and to decrease the effective concentrations of this antibiotic for several orders.12 We effectively designed texture and defects on material surface to optimize antimicrobial effect 13 and combined antimicrobial effects of nano-Ga and functionalized nano-gold into very effective antimicrobial technology.14 Our preliminary ongoing research also confirm applicability of piezo-PLLA and laser-activated ferrite-AuNPs against bacterial biofilms.
3. Unique methodology
Novel method for more effective functionalization of AuNPs will be developed using preformed amino acid – cyclodextrin inclusion complexes. Highly uniform, mono-dispersed GaNPs will be formed in inert atmosphere (using Schlenk line) which will be combined with novel procedure for surface ligands exchange that will enable effective formation of core-shell structures. In addition to melting-assisted stretching, we will work on processing piezo-PLLA layers using epitaxial growth as well as 3D printing.
VIDEO: Cooperation of JSI with SMEs within KETGATE project
Success Story

REFERENCES:
1 N. F. Kamaruzzaman, Br J Pharmacol. 174(14) (2017) 2225.
2 Y. F. Dufrêne, A. Persat, Nat Rev Microbiol (2020).
3 R. Sender et al., PLoS Biol. 2016 Aug; 14(8): e1002533.
4 J. Libertucci & V. B. Young, Nature Microbiol. 4, 35–45(2019)
5 M. M. Fernandes, et al. Frontiers Bioeng. Biotechnol., 7 (2019) 277
6 M. Lomazzi et al., BMC Public Health, 19 (2019) 858
7 J.-W. Xu et al., Nanoscale, 2019, 11, 8680
8 T. F. C. Mah and G. A. O’Toole, Trends Microbiol. 9 (2001) 34–39
9 D. L. Worcester, D. L. Proc. Natl. Acad. Sci. U.S.A. 75 (1978) 5475–5477
10 D. Miklavcic, et al. Bioelectrochemistry 63 (2004) 311–315.
11 M. Vukomanovic, S. Skapin, D. Suvorov, EP2863751A1, P-201200204
12 M. Vukomanović et al. Sci. Rep. 7 (2017) 4324. ARTICLE DOWNLOAD
13 M. Vukomanović et al. Small, 2018, 1800205. ARTICLE DOWNLOAD
14 M. Kurtjak, M. Vukomanovic, D. Suvorov, Mater. Lett. 193 (2017) 126. ARTICLE DOWNLOAD
15 World Health Organization (WHO), (2014), http://www.who.int/mediacentre/factsheets/fs310/en/
16 M. Haswell, Implant Practice, 2 (2009) 44.
17 M. Manco, et al, Endocrine Rev. 31 (2010) 817–844.
18 J. Libertucci & V. B. Young, Nature Microbiol. 4, 35–45(2019)
